Omega-3 Supplements Guide: Eicosapentaenoic Acid Benefits and Clinical Evidence
🎯 Key Takeaway
High-quality omega-3 supplements containing eicosapentaenoic acid (EPA) demonstrate significant cardiovascular benefits in clinical trials. Furthermore, EPA-dominant formulations show superior outcomes compared to combined EPA/DHA supplements, consequently making EPA the preferred choice for heart health optimization.
The landscape of cardiovascular health supplementation has been dramatically reshaped by mounting evidence supporting omega-3 fatty acids, particularly eicosapentaenoic acid. Moreover, recent landmark clinical trials have provided unprecedented clarity regarding optimal omega-3 formulations and dosing strategies. Nevertheless, the supplement market remains flooded with varying formulations, consequently creating confusion among healthcare providers and consumers alike.
Understanding which omega-3 supplements provide genuine clinical benefits requires careful examination of the scientific evidence. Additionally, distinguishing between prescription-grade formulations and standard dietary supplements becomes essential for achieving therapeutic outcomes. Comprehensive cardiovascular risk management increasingly incorporates targeted omega-3 supplementation as evidence-based therapy rather than general wellness support.
Understanding Eicosapentaenoic Acid: The Primary Omega-3 Powerhouse
Eicosapentaenoic acid represents one of the three clinically significant omega-3 fatty acids, alongside docosahexaenoic acid (DHA) and alpha-linolenic acid (ALA). Furthermore, EPA’s unique molecular structure enables specific cardiovascular benefits that distinguish it from other omega-3 compounds. Cleveland Clinic research indicates that EPA’s anti-inflammatory and membrane-stabilizing properties provide targeted cardiovascular protection.
The biochemical mechanisms underlying EPA’s cardiovascular benefits involve multiple pathways. Specifically, research published in Lipids in Health and Disease demonstrates that EPA modulates inflammatory cascades, improves endothelial function, and stabilizes atherosclerotic plaques through distinct molecular mechanisms.
EPA’s Molecular Mechanisms of Action
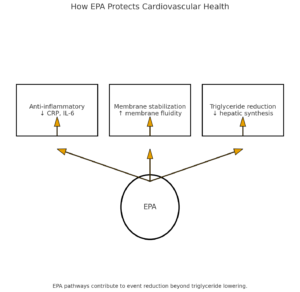
⏳ How EPA Protects Cardiovascular Health
Anti-Inflammatory Pathway Modulation
Initially, EPA competes with arachidonic acid for enzymatic conversion, therefore reducing pro-inflammatory mediator production. Subsequently, this process decreases systemic inflammation markers including C-reactive protein and interleukin-6.
Membrane Stabilization Effects
Additionally, EPA incorporates into cell membrane phospholipids, consequently improving membrane fluidity and cellular function. Therefore, this stabilization enhances cardiomyocyte function and reduces arrhythmia risk.
Triglyceride Reduction Mechanisms
Furthermore, EPA inhibits hepatic triglyceride synthesis while enhancing fatty acid oxidation. Consequently, this dual mechanism produces significant triglyceride reductions, particularly beneficial for individuals with hypertriglyceridemia.
Importantly, recent meta-analyses published in Circulation Research confirm that EPA’s cardiovascular benefits extend beyond simple triglyceride reduction. Nevertheless, the optimal EPA dosing and formulation continue to evolve based on emerging clinical evidence.
FDA-Approved Omega-3 Formulations and Regulatory Landscape

The regulatory environment surrounding omega-3 supplements has undergone significant evolution, particularly following breakthrough clinical trial results. Moreover, the FDA’s recent qualified health claims for EPA and DHA represent a major shift in regulatory recognition of omega-3 cardiovascular benefits.
Currently, several FDA-approved prescription omega-3 medications provide high-potency EPA formulations. Specifically, StatPearls medical references detail that icosapent ethyl (Vascepa) contains purified EPA and demonstrates superior cardiovascular outcomes compared to combination EPA/DHA formulations.
Prescription vs. Dietary Supplement Formulations
| Formulation Type | EPA Content | Purity Standards | Clinical Evidence | Regulatory Oversight |
|---|---|---|---|---|
| Prescription Icosapent Ethyl | 1,000mg per capsule | >96% pure EPA | REDUCE-IT trial validated | Full FDA approval |
| Prescription EPA/DHA | 465mg EPA + 375mg DHA | Pharmaceutical grade | Mixed clinical results | FDA approved |
| High-Quality Supplements | Variable (300-800mg) | Third-party tested | Limited clinical data | Dietary supplement |
| Standard Fish Oil | Variable (180-300mg) | Basic standards | Inconsistent evidence | Minimal oversight |
The distinction between prescription and supplement formulations becomes particularly important when considering therapeutic applications. Additionally, industry analyses suggest that pharmaceutical-grade omega-3 products provide more consistent clinical outcomes due to standardized manufacturing and quality control processes.
Landmark Clinical Trials: REDUCE-IT and Beyond
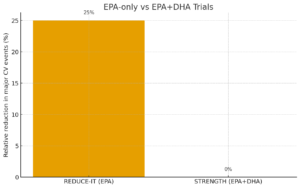
The cardiovascular benefits of EPA supplementation gained unprecedented validation through the REDUCE-IT trial, consequently establishing EPA as a evidence-based therapeutic intervention. Furthermore, this landmark study demonstrated that high-dose EPA supplementation produces clinically meaningful cardiovascular risk reductions in statin-treated patients.
Comprehensive analysis of recent omega-3 trials reveals important distinctions between EPA-only formulations and combination EPA/DHA products. Specifically, trials using purified EPA consistently demonstrate superior cardiovascular outcomes compared to combination formulations.
Major Omega-3 Clinical Trials Comparison
REDUCE-IT Trial (EPA-Only)
Population: 8,179 patients with elevated triglycerides on statin therapy
Intervention: 4g daily purified EPA (icosapent ethyl)
Results: 25% reduction in major cardiovascular events, 20% reduction in cardiovascular death
Significance: Led to FDA approval for cardiovascular risk reduction
STRENGTH Trial (EPA+DHA)
Population: 13,078 patients with elevated cardiovascular risk
Intervention: 4g daily EPA+DHA combination
Results: No significant reduction in cardiovascular events
Significance: Trial terminated early due to futility
The contrasting results between REDUCE-IT and STRENGTH trials highlight the importance of EPA purity in omega-3 formulations. Moreover, recent meta-analyses published in eClinicalMedicine confirm that EPA-dominant formulations provide superior cardiovascular protection compared to balanced EPA/DHA combinations.
VITAL Trial: Primary Prevention Evidence
The Vitamin D and Omega-3 Trial (VITAL) provided crucial evidence for omega-3 supplementation in primary prevention settings. Additionally, this massive study involving over 25,000 participants demonstrated that moderate-dose omega-3 supplementation reduces heart attack risk by 28% in healthy individuals.
The VITAL trial results suggest that omega-3 fatty acids, particularly EPA, provide cardiovascular benefits even in primary prevention settings, though the optimal dosing and formulation continue to be refined through ongoing research.
Importantly, Mayo Clinic Proceedings analysis indicates that cardiovascular benefits appear dose-dependent, with higher EPA intake producing more significant risk reductions. Nevertheless, optimal dosing strategies must balance cardiovascular benefits with potential adverse effects.
Optimal Dosing Strategies and Clinical Applications
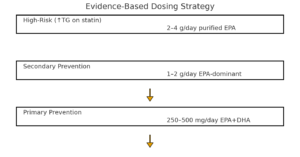
Determining appropriate omega-3 supplementation dosing requires consideration of individual cardiovascular risk factors, existing medical conditions, and therapeutic goals. Furthermore, recent clinical evidence suggests that traditional “one-size-fits-all” dosing approaches may be insufficient for achieving optimal cardiovascular protection.
The National Institutes of Health Office of Dietary Supplements provides comprehensive guidance on omega-3 dosing based on current clinical evidence. Nevertheless, individualized dosing approaches often produce superior outcomes compared to standard recommendations.
Evidence-Based Dosing Guidelines
⏳ Progressive Dosing Strategy
Primary Prevention (Low Risk)
Initially, healthy individuals should aim for 250-500mg combined EPA/DHA daily through dietary sources or supplements. Subsequently, this baseline intake supports general cardiovascular health while minimizing intervention costs.
Secondary Prevention (Established CVD)
Additionally, individuals with established cardiovascular disease benefit from 1-2 grams daily of EPA-dominant formulations. Therefore, this therapeutic dosing level provides meaningful cardiovascular risk reduction based on clinical trial evidence.
High-Risk Populations
Furthermore, patients with elevated triglycerides (>135 mg/dL) and statin use may require 2-4 grams daily of purified EPA. Consequently, this intensive dosing strategy mirrors the REDUCE-IT trial protocol and maximizes cardiovascular protection.
Recent research indicates that omega-3 blood testing provides the most accurate method for determining individual supplementation needs. Specifically, targeting an omega-3 index above 8% appears optimal for cardiovascular protection, though achieving this level often requires higher doses than traditionally recommended.
Safety Profile and Potential Adverse Effects
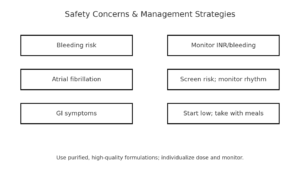
While omega-3 supplements generally demonstrate excellent safety profiles, recent large-scale clinical trials have identified specific adverse effects that require careful consideration. Moreover, the safety profile varies significantly based on dosing levels, formulation types, and individual patient characteristics.
❌ Common Safety Concerns
- Increased bleeding risk with anticoagulant medications
- Elevated atrial fibrillation incidence in some populations
- Gastrointestinal symptoms including nausea and diarrhea
- Potential immune function suppression at very high doses
✅ Safety Management Strategies
- Monitor bleeding parameters in anticoagulated patients
- Screen for atrial fibrillation risk factors before initiation
- Start with lower doses and gradually increase as tolerated
- Use high-quality, purified formulations to minimize contaminants
The REDUCE-IT trial provided valuable safety data for high-dose EPA supplementation. Specifically, Harvard Medical School analysis indicates that while cardiovascular benefits are substantial, atrial fibrillation risk increases from 3.9% to 5.3% with high-dose EPA supplementation.
Drug Interactions and Contraindications
Understanding potential omega-3 interactions with common cardiovascular medications becomes essential for safe supplementation. Additionally, certain medical conditions may contraindicate high-dose omega-3 supplementation or require enhanced monitoring protocols.
| Medication Class | Interaction Type | Clinical Significance | Management Strategy |
|---|---|---|---|
| Anticoagulants (Warfarin) | Additive bleeding risk | Moderate | Monitor INR closely, consider dose adjustment |
| Antiplatelet Agents | Enhanced bleeding potential | Moderate | Monitor for bleeding signs, start low dose |
| Blood Pressure Medications | Additive hypotensive effect | Mild | Monitor blood pressure regularly |
| Statins | Synergistic benefits | Positive | Maintain both therapies as indicated |
Importantly, comprehensive safety reviews indicate that most omega-3 adverse effects are dose-dependent and reversible. Nevertheless, careful patient selection and monitoring protocols ensure optimal safety outcomes while maximizing cardiovascular benefits.
Quality Considerations and Supplement Selection
The omega-3 supplement market contains significant variability in product quality, purity, and potency. Furthermore, manufacturing processes, storage conditions, and source materials dramatically influence supplement effectiveness and safety profiles. Recent regulatory compliance studies reveal concerning inconsistencies in omega-3 supplement labeling and content accuracy.
✅ Quality Assessment Criteria
- Third-party testing: Independent verification of EPA/DHA content and purity levels
- Molecular distillation: Removal of heavy metals, PCBs, and other environmental contaminants
- Oxidation resistance: Proper storage and antioxidant addition to prevent rancidity
- Bioavailability optimization: Formulation design to enhance absorption and utilization
- Sustainable sourcing: Environmentally responsible fishing and manufacturing practices
Recent advances in omega-3 formulation technology have produced novel delivery systems that enhance bioavailability. Additionally, emerging research on polar lipid formulations suggests that certain processing methods can significantly improve EPA absorption and utilization compared to traditional ethyl ester formulations.
Formulation Types and Bioavailability
Ethyl Ester Formulations
Most common supplement form with moderate bioavailability. Furthermore, these formulations require gastric acid for optimal absorption, consequently performing better when taken with meals containing dietary fats.
Triglyceride Formulations
Natural fish oil form with enhanced bioavailability compared to ethyl esters. Additionally, these formulations resist oxidation better and provide more consistent absorption across diverse populations.
The choice between formulation types significantly impacts therapeutic outcomes. Moreover, clinical studies comparing different formulations demonstrate that polar lipid and re-esterified triglyceride forms provide superior bioavailability compared to standard ethyl ester supplements.
Integration with Comprehensive Cardiovascular Care
Omega-3 supplementation represents one component of evidence-based cardiovascular risk reduction strategies. Furthermore, optimal outcomes require integration with lifestyle modifications, appropriate pharmaceutical interventions, and ongoing medical monitoring. Comprehensive cholesterol management increasingly incorporates targeted omega-3 therapy as part of personalized treatment approaches.
The integration of high-dose EPA supplementation with statin therapy represents a paradigm shift in cardiovascular risk management, offering unprecedented opportunities for residual risk reduction in high-risk populations.
Current clinical guidelines increasingly recognize omega-3 supplementation as evidence-based therapy rather than general wellness support. Specifically, recent clinical practice guidelines recommend EPA supplementation for specific patient populations based on rigorous clinical trial evidence.
Strategic Implementation in Clinical Practice
⏳ Clinical Decision-Making Framework
Risk Assessment and Stratification
Initially, healthcare providers should evaluate cardiovascular risk factors, including lipid profiles, blood pressure, and family history. Subsequently, this assessment determines whether omega-3 supplementation provides meaningful clinical benefits for individual patients.
Formulation Selection and Dosing
Additionally, selecting appropriate omega-3 formulations requires consideration of patient-specific factors including medication interactions, bleeding risk, and therapeutic goals. Therefore, individualized approaches often produce superior outcomes compared to standardized protocols.
Monitoring and Optimization
Furthermore, ongoing monitoring of cardiovascular biomarkers, safety parameters, and clinical outcomes enables optimization of omega-3 therapy. Consequently, this iterative approach maximizes benefits while minimizing potential adverse effects.
The future of omega-3 supplementation likely involves increasingly personalized approaches based on genetic testing, biomarker profiles, and individual response patterns. Nevertheless, current evidence provides sufficient guidance for evidence-based omega-3 utilization in cardiovascular risk management.
Emerging Research and Future Directions
The field of omega-3 research continues evolving rapidly, with emerging studies exploring novel applications, optimal dosing strategies, and innovative formulations. Moreover, ongoing clinical trials investigate EPA supplementation for neurological conditions, inflammatory disorders, and cancer prevention applications.
Recent breakthroughs in omega-3 research include the development of algae-based EPA sources, enhanced bioavailability formulations, and precision dosing based on genetic polymorphisms. Additionally, innovative polar lipid formulations demonstrate superior cardiovascular benefits compared to traditional fish oil supplements.
Areas of Active Investigation
Precision Medicine Applications
Genetic testing for omega-3 metabolism variants may enable personalized dosing strategies. Furthermore, biomarker-guided therapy could optimize individual responses while minimizing unnecessary supplementation costs.
Novel Therapeutic Applications
Beyond cardiovascular health, EPA supplementation shows promise for depression treatment, cognitive function enhancement, and inflammatory condition management based on emerging clinical evidence.
The integration of artificial intelligence and machine learning in omega-3 research promises to accelerate discovery of optimal therapeutic applications. Nevertheless, rigorous clinical validation remains essential before new applications can be recommended for clinical practice.
References
- Bhatt, D. L., et al. (2019). Recent Clinical Trials Shed New Light on the Cardiovascular Benefits of Omega-3 Fatty Acids. Circulation Research, 124(5), 637-641.
- Mason, R. P., & Sherratt, S. C. (2017). Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids in Health and Disease, 16, 23.
- U.S. Food and Drug Administration. (2024). FDA Announces New Qualified Health Claims for EPA and DHA Omega-3 Consumption. FDA Consumer Updates.
- National Center for Biotechnology Information. (2024). Omega-3 Fatty Acids. StatPearls.
- Khan, S. U., et al. (2021). Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine, 38, 100997.
- Bernasconi, A. A., et al. (2021). Effect of Omega-3 Dosage on Cardiovascular Outcomes. Mayo Clinic Proceedings, 96(2), 304-313.
- National Institutes of Health. (2024). Omega-3 Fatty Acids – Health Professional Fact Sheet. Office of Dietary Supplements.
- Cleveland Clinic. (2024). Omega-3 Fatty Acids & the Important Role They Play. Cleveland Clinic Health Library.
- Ganuza, E., et al. (2024). Omega-3 eicosapentaenoic polar-lipid rich extract from microalgae Nannochloropsis decreases plasma triglycerides and cholesterol. Frontiers in Nutrition, 11, 1293909.
- Kelley-Hedgepeth, A. (2021). Omega-3 fatty acids and the heart: New evidence, more questions. Harvard Health Blog.
- Nordic Naturals. (2024). Omega-3 Dosage: How Much EPA and DHA Should I Take? Nordic Naturals Science.
- Nutritional Medicine Institute. (2024). EPA / DHA: A Review of Clinical Use and Efficacy. NMI Health.
- Djuricic, I., & Calder, P. C. (2021). Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine, 38, 100997.
- Weihrauch, D., et al. (2024). Regulatory Compliance of Health Claims on Omega-3 Fatty Acid Food Supplements. Foods, 14(1), 67.
- Ghasemi Fard, S., et al. (2020). Omega-3 Eicosapentaenoic Acid (EPA) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals. Nutrients, 12(6), 1869.
Related Articles
- High Cholesterol: Complete Guide to Understanding and Managing High LDL Levels
- Omega-3 vs Omega-6: Finding the Right Balance to Reduce Inflammation(Opens in a new browser tab)
- Lower Cholesterol Supplements that actually work(Opens in a new browser tab)
Author Bio
The Remedy Verified Team translates complex metabolic science into clear, practical strategies for everyday health.
Disclaimer
This article is for educational purposes only and should not replace professional medical advice. Always consult with qualified healthcare providers before starting omega-3 supplementation, especially if you have existing medical conditions, take medications, or are at risk for bleeding disorders. Individual responses to supplementation may vary, and therapeutic outcomes depend on multiple factors including dosing, formulation quality, and overall health status.