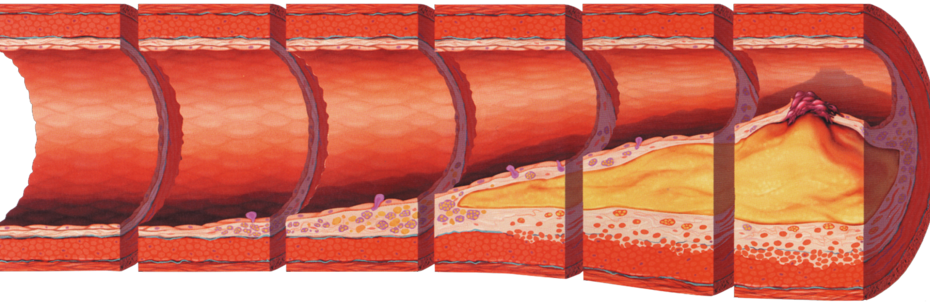
High Cholesterol: Complete Guide to Understanding and Managing High LDL Levels
High cholesterol has earned its reputation as the “silent killer” for good reason—it typically causes no symptoms while quietly building dangerous plaque deposits in your arteries for decades. However, despite decades of public health campaigns, recent 2025 guidelines show that optimal cholesterol management remains elusive for millions of people, with many still unaware of their elevated risk.
Remarkably, the landscape of cholesterol management has evolved dramatically in recent years. What once focused primarily on dietary cholesterol restriction has shifted toward a more sophisticated understanding of how different types of cholesterol particles behave in your body. Furthermore, we now better understand why some people develop atherosclerosis faster than others, and which interventions provide the greatest protective benefit.
The Cholesterol Transport System: Beyond Good vs. Bad
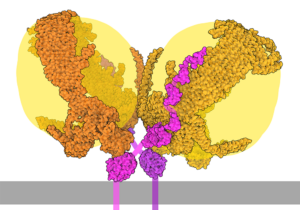
To understand why high cholesterol poses such a significant threat, we need to examine how your body transports this essential but potentially dangerous molecule. Cholesterol is fundamentally a waxy, fatty substance that serves critical functions—building cell membranes, producing hormones like testosterone and estrogen, and creating bile acids for digestion.
Since cholesterol can’t dissolve in water-based blood, your body packages it into spherical particles called lipoproteins. Essentially, these microscopic “delivery trucks” consist of a protein shell surrounding a core of cholesterol and triglycerides, allowing safe transport through your bloodstream.
The two primary players in this transport system are:
Low-Density Lipoprotein (LDL)
Function: Delivers cholesterol from liver to tissues
Risk Factor: High levels contribute to arterial plaque buildup
Optimal Level: Below 100 mg/dL (below 70 mg/dL for high-risk individuals)
High-Density Lipoprotein (HDL)
Function: Transports cholesterol back to liver for disposal
Protective Factor: Higher levels associated with reduced cardiovascular risk
Optimal Level: Above 40 mg/dL (men), above 50 mg/dL (women)
The Atherosclerosis Mechanism: How High LDL Damages Arteries
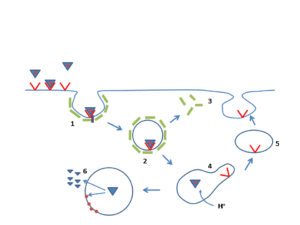
The process by which high cholesterol leads to cardiovascular disease involves a complex cascade of events that begins long before symptoms appear. Moreover, recent research from the National Institute of Health has revealed the precise molecular mechanisms underlying this process.
⏳ Atherosclerosis Development Timeline
Stage 1: Endothelial Dysfunction (Years 1-5)High LDL particles infiltrate arterial walls, particularly at sites of disturbed blood flow like arterial bifurcations. The “response-to-retention” concept shows that LDL particles become trapped in the subendothelial space, where they undergo oxidative modifications.
Stage 2: Inflammatory Response (Years 5-15)Oxidized LDL triggers immune system activation, attracting monocytes that differentiate into macrophages. These cells engulf oxidized LDL particles, forming characteristic “foam cells” that create visible fatty streaks in arteries.
Stage 3: Plaque Formation (Years 15-30)Smooth muscle cells migrate from the arterial media, proliferate, and produce extracellular matrix. This creates a fibrous cap over the lipid core, forming mature atherosclerotic plaques that narrow arterial lumens.
Stage 4: Plaque Instability (Years 20-40)Advanced plaques may rupture, exposing thrombogenic material to flowing blood. This triggers rapid clot formation, potentially causing complete arterial occlusion and resulting in heart attack or stroke.
“The key initiating event in atherogenesis is the retention of cholesterol-rich apolipoprotein B-containing lipoproteins within the arterial wall, particularly in the presence of endothelial dysfunction.”
Risk Assessment and Current Guidelines
The approach to cholesterol management has undergone significant refinement since the landmark 2018 AHA/ACC/Multi-Society Cholesterol Guidelines. Rather than focusing solely on cholesterol numbers, current protocols emphasize comprehensive cardiovascular risk assessment.
Primary Prevention Risk Categories
| Risk Category | 10-Year ASCVD Risk | LDL Target | Treatment Approach |
|---|---|---|---|
| Low Risk | <5% | <100 mg/dL | Lifestyle optimization |
| Borderline Risk | 5-7.4% | <100 mg/dL | Lifestyle + risk enhancers consideration |
| Intermediate Risk | 7.5-19.9% | <100 mg/dL | Moderate-intensity statin + lifestyle |
| High Risk | ≥20% | <70 mg/dL | High-intensity statin + lifestyle |
Risk-Enhancing Factors
Modern risk assessment incorporates additional factors that can reclassify patients to higher risk categories, warranting more aggressive treatment. Consequently, these factors have become crucial in determining optimal treatment approaches.
Metabolic Risk Enhancers: Family history of premature ASCVD (men <55, women <65 years), primary hypercholesterolemia with LDL ≥160 mg/dL, chronic kidney disease (eGFR 15-59 mL/min/1.73m²), metabolic syndrome (≥3 of 5 criteria), and chronic inflammatory conditions (rheumatoid arthritis, psoriasis).
Advanced Biomarkers: Additionally, high-sensitivity CRP ≥3.0 mg/L, Lipoprotein(a) ≥50 mg/dL (125 nmol/L), Apolipoprotein B ≥130 mg/dL, and Ankle-brachial index <0.9 provide valuable risk stratification data.
Advanced Cholesterol Testing: Beyond the Basic Panel
While standard lipid panels provide valuable baseline information, advanced testing can reveal critical details about cardiovascular risk that traditional measurements miss. Recent expert consensus from the American College of Cardiology emphasizes the importance of comprehensive lipid profiling in high-risk patients.
Advanced Cholesterol Testing: Beyond the Basic Panel
Coronary Artery Calcium Scoring
For patients at borderline or intermediate risk, coronary artery calcium (CAC) scoring provides powerful additional risk stratification. This non-invasive CT scan measures calcified plaque in coronary arteries, offering a direct window into atherosclerotic burden.
CAC Score Interpretation: 0 indicates very low risk – consider lifestyle optimization; 1-99 shows mild plaque – consider statin therapy; 100-399 reveals moderate plaque – statin therapy recommended; ≥400 demonstrates extensive plaque – high-intensity statin therapy indicated.
Clinical Applications: Furthermore, CAC >0 in borderline risk patients often warrants statin initiation for upward risk reclassification. Conversely, CAC = 0 in intermediate risk patients may allow lifestyle-only approach for downward risk reclassification.
Lifestyle Interventions: The Foundation of Cholesterol Management
Comprehensive lifestyle modification remains the cornerstone of cholesterol management and can achieve LDL reductions of 20-30% in motivated individuals. Nevertheless, the approach has evolved from simple “low-fat” recommendations to sophisticated protocols targeting multiple mechanisms of cholesterol metabolism.

Evidence-Based Dietary Strategies
Research from Dr. David Jenkins demonstrates that combining multiple cholesterol-lowering foods can achieve LDL reductions comparable to low-dose statin therapy. Specifically, this “dietary portfolio” includes plant sterols, soluble fiber, soy protein, and tree nuts.
Foods to Emphasize
- Soluble fiber sources: Oats, barley, beans, apples, citrus fruits (10-25g daily)
- Plant sterols/stanols: Fortified margarine, nuts, seeds (2g daily)
- Omega-3 rich fish: Salmon, mackerel, sardines (2-3 servings weekly)
- Tree nuts: Almonds, walnuts, pistachios (1-2 oz daily)
- Soy protein: Tofu, tempeh, soy milk (25g daily)
Foods to Limit
- Saturated fats: Fatty meats, full-fat dairy, tropical oils (<7% of calories)
- Trans fats: Partially hydrogenated oils, fried foods (eliminate completely)
- Refined carbohydrates: White bread, sugary drinks, processed snacks
- Excess calories: Portion control for weight management
Exercise Protocols for Optimal Lipid Effects
Cleveland Clinic research shows that different exercise modalities provide distinct benefits for cholesterol management.
Aerobic Exercise: Primarily, the main benefit is raising HDL cholesterol. Recommended dose: 150-300 minutes moderate intensity weekly. Example activities include brisk walking, cycling, and swimming.
Resistance Training: Meanwhile, the primary benefit is improving LDL particle size distribution. Recommended dose: 2-3 sessions weekly, major muscle groups. Example activities include weight lifting, resistance bands, and bodyweight exercises.
Emerging research suggests HIIT may provide superior lipid benefits compared to steady-state cardio. Typically, a standard protocol involves 4-minute high-intensity intervals (85-95% max heart rate) alternated with 3-minute recovery periods, performed 2-3 times weekly.
Pharmacological Management: Current and Emerging Therapies
When lifestyle interventions prove insufficient, pharmacological therapy becomes essential. Furthermore, The 2025 AACE Clinical Practice Guidelines provide updated recommendations incorporating newer therapeutic options with proven cardiovascular outcomes benefits.
Statin Therapy: The Gold Standard
Statins remain the first-line pharmacological intervention due to extensive evidence demonstrating both LDL lowering and cardiovascular event reduction. Mechanistically, these medications work by inhibiting HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis.
| Intensity | Medication | LDL Reduction | Clinical Indications |
|---|---|---|---|
| High-Intensity | Atorvastatin 40-80mg Rosuvastatin 20-40mg | ≥50% | ASCVD, LDL ≥190, diabetes ages 40-75 |
| Moderate-Intensity | Atorvastatin 20mg Rosuvastatin 10mg Simvastatin 20-40mg | 30-49% | Primary prevention, intermediate risk |
| Low-Intensity | Simvastatin 10mg Pravastatin 10-20mg | <30% | Statin intolerance, elderly patients |
Non-Statin Therapies: Expanding the Arsenal
Ezetimibe: The Cholesterol Absorption Inhibitor – Ezetimibe blocks cholesterol absorption in the small intestine, providing an additional 15-20% LDL reduction
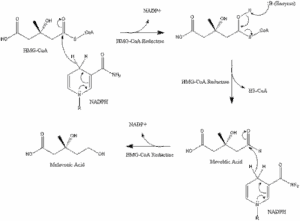
when added to statin therapy. Notably, the landmark IMPROVE-IT trial demonstrated cardiovascular outcomes benefits in post-acute coronary syndrome patients.
PCSK9 Inhibitors: Revolutionary LDL Lowering – These monoclonal antibodies (evolocumab, alirocumab) or small interfering RNA (inclisiran) dramatically reduce LDL by 50-60% beyond statin therapy. Currently, they’re reserved for very high-risk patients due to cost considerations, yet they’ve shown impressive cardiovascular event reduction in outcome trials.
🔬 Emerging Cholesterol Therapies
Bempedoic AcidOral medication that inhibits cholesterol synthesis upstream of statins. Provides 15-25% LDL reduction with lower risk of muscle-related side effects.
InclisiranSmall interfering RNA administered twice yearly by subcutaneous injection. Offers durable LDL reductions of 50%+ with convenient dosing schedule.
EvinacumabANGPTL3 inhibitor approved for homozygous familial hypercholesterolemia. Represents novel mechanism targeting lipoprotein metabolism.
Special Populations and Complex Cases
Familial Hypercholesterolemia: Genetic High Cholesterol
Familial hypercholesterolemia affects 1 in 200-250 individuals, making it one of the most common genetic disorders. These patients exhibit markedly elevated LDL cholesterol from birth, often exceeding 190 mg/dL despite optimal lifestyle management.
⚠️ Recognizing Familial Hypercholesterolemia
Clinical signs: Tendon xanthomas, corneal arcus before age 45, family history of premature cardiovascular disease
Laboratory findings: LDL ≥190 mg/dL in adults, ≥160 mg/dL in children
Treatment urgency: Requires immediate high-intensity statin therapy, often combination treatment
❌ Myth
Young people with familial hypercholesterolemia can wait until adulthood to start treatment.
✅ Fact
Early intervention is crucial. Studies show that every decade of delay in treatment increases cardiovascular risk by 30-50%. Statin therapy is safe and effective in children as young as 8-10 years old.
Women and Cholesterol: Lifecycle Considerations
Cholesterol management in women requires consideration of hormonal fluctuations, pregnancy, and menopause. Estrogen generally provides cardiovascular protection through favorable effects on lipid profiles, but this protection diminishes after menopause.
Pre-Menopausal Considerations: Lower baseline cardiovascular risk, statin use requires contraception counseling, pregnancy necessitates statin discontinuation, and focus on lifestyle optimization.
Post-Menopausal Changes: LDL cholesterol typically increases 10-15%, HDL cholesterol may decline, cardiovascular risk approaches male levels, and statin therapy often becomes appropriate.
Monitoring and Long-Term Management
Effective cholesterol management requires systematic monitoring and periodic reassessment of treatment strategies. According to current guidelines, lipid testing should occur every 4-6 years for low-risk adults, with more frequent monitoring for those on therapy or at higher risk.
Treatment Response Assessment
Initial Monitoring Protocol
✅ Baseline lipid panel before initiating therapy
✅ Safety laboratories (liver enzymes, creatine kinase) if using statins
✅ 4-6 week follow-up after starting medication
✅ Dose optimization based on response and tolerability
✅ Annual monitoring once stable on therapy
If LDL goals aren’t achieved on maximally tolerated statin therapy, consider adding ezetimibe first (most cost-effective), followed by PCSK9 inhibitors for very high-risk patients requiring additional LDL reduction.
Managing Statin Intolerance
Approximately 10-15% of patients experience statin-related side effects, most commonly muscle symptoms. However, many patients labeled as “statin intolerant” can successfully resume therapy with appropriate management strategies.
Step 1: Confirm True Intolerance – Initially, discontinue statin for 2-4 weeks to ensure symptom resolution. Additionally, rule out other causes of muscle symptoms (hypothyroidism, vitamin D deficiency).
Step 2: Rechallenge Strategy – Subsequently, try alternative statin (different class), lower dose, or alternate-day dosing. Consider coenzyme Q10 supplementation for muscle symptoms.
Step 3: Non-Statin Options – Finally, if rechallenge fails, initiate ezetimibe, bempedoic acid, or PCSK9 inhibitors based on cardiovascular risk and cost considerations.
Cutting-Edge Research and Future Directions
The field of cholesterol management continues evolving rapidly, with several promising therapeutic approaches in development. Understanding these emerging strategies provides insight into the future of cardiovascular prevention.
Novel Therapeutic Targets
Investigational Approaches: CETP Inhibitors block cholesteryl ester transfer protein to raise HDL and lower LDL. APOC3 Antisense therapy targets apolipoprotein C3 to reduce triglyceride-rich lipoproteins. Lipoprotein(a) Reduction uses antisense oligonucleotides specifically targeting Lp(a) production. Base Editing employs gene editing techniques to permanently modify cholesterol metabolism genes.
Precision Medicine in Lipid Management
Genetic testing is increasingly informing cholesterol treatment decisions. Polygenic risk scores combining multiple genetic variants may identify individuals who benefit most from intensive LDL lowering, while pharmacogenetic testing can predict statin response and toxicity risk.
Pharmacogenetic considerations include SLCO1B1 variants that affect statin uptake into hepatocytes, influencing both efficacy and myopathy risk. CYP2C9 variants impact metabolism of certain statins, affecting appropriate dosing. HMGCR variants influence natural cholesterol production and statin response.
Practical Implementation: Patient-Centered Protocols

Translating evidence-based guidelines into clinical practice requires individualized approaches that account for patient preferences, adherence challenges, and real-world constraints. The most effective strategies combine optimal medical therapy with sustainable lifestyle modifications.
Shared Decision-Making Framework
Patient Education Topics: Absolute vs. relative risk reduction, number needed to treat concepts, expected benefits and potential harms, alternative treatment strategies, and cost-effectiveness considerations.
Treatment Personalization: Individual cardiovascular risk level, comorbidity considerations, medication preferences and concerns, lifestyle modification capacity, and monitoring and follow-up preferences.
Adherence Optimization Strategies
Enhancing medication adherence involves simplifying regimens with once-daily dosing when possible, addressing cost barriers through generic options and patient assistance programs, monitoring for side effects with proactive management to prevent discontinuation, providing education materials with written summaries of treatment rationale, and using technology tools like apps, reminders, and telehealth monitoring.
“The greatest risk to patients is not statin side effects, but rather the cardiovascular events that occur when high-risk patients discontinue effective therapy.”
Common Myths and Misconceptions
Despite decades of research, numerous misconceptions about cholesterol persist among patients and even some healthcare providers. Therefore, addressing these myths is crucial for optimal patient care and adherence to evidence-based treatments.
❌ Myth
Dietary cholesterol is the primary driver of blood cholesterol levels.
✅ Fact
Only about 20% of blood cholesterol comes from diet—the liver produces the remaining 80%. Saturated and trans fats have much greater impact on LDL levels than dietary cholesterol itself.
❌ Myth
Statins cause widespread muscle damage and cognitive decline.
✅ Fact
Large randomized controlled trials show that true statin-induced myopathy occurs in less than 1% of patients. Cognitive effects are rare and reversible. The cardiovascular benefits far outweigh these uncommon risks for appropriate candidates.
❌ Myth
Very low LDL cholesterol levels are dangerous.
✅ Fact
Multiple studies demonstrate that LDL levels as low as 30-40 mg/dL are safe and provide additional cardiovascular benefits. There appears to be no threshold below which further LDL reduction becomes harmful.
Global Perspectives and Healthcare Disparities
Cholesterol management strategies vary significantly worldwide, influenced by healthcare systems, medication access, cultural factors, and genetic variations among populations. Understanding these differences is crucial for addressing health disparities and improving global cardiovascular outcomes.
High-Income Countries: Widespread statin availability and use, advanced lipid testing capabilities, specialized lipid clinics for complex cases, and PCSK9 inhibitor access for high-risk patients.
Low- and Middle-Income Countries: Limited access to cholesterol testing, cost barriers to statin therapy, focus on generic medication options, and emphasis on lifestyle interventions.
Certain populations face disproportionately high cholesterol levels and cardiovascular risk, including African Americans, Hispanic/Latino Americans, and individuals with lower socioeconomic status. Culturally appropriate interventions, community health programs, and policy initiatives are essential for reducing these disparities.
The Economic Impact of Cholesterol Management
The economic implications of cholesterol management extend far beyond medication costs, encompassing healthcare utilization, productivity, and quality of life considerations. Cost-effectiveness analyses consistently demonstrate that appropriate cholesterol management provides excellent value for healthcare systems.
Without Optimal Cholesterol Management: Higher rates of heart attacks and strokes, increased hospitalizations and procedures, greater disability and lost productivity, and higher long-term healthcare costs.
With Effective Cholesterol Control: Significant reduction in cardiovascular events, lower hospitalization rates, improved quality of life and longevity, and reduced overall healthcare expenditure.
Conclusion: A Roadmap to Cardiovascular Health
High cholesterol remains one of the most important modifiable risk factors for cardiovascular disease, but our understanding of optimal management continues evolving rapidly. Indeed, the shift from simple cholesterol targets to comprehensive risk assessment, the introduction of powerful new medications, and the recognition of lifestyle medicine’s crucial role have revolutionized the field.
🎯 Key Takeaways for Patients
Know your numbers: Regular cholesterol testing is essential for risk assessment
Embrace lifestyle medicine: Diet, exercise, and weight management provide substantial benefits
Don’t fear evidence-based medications: Statins and other therapies save lives when used appropriately
Work with your healthcare team: Individualized treatment plans optimize outcomes
Stay informed: The field continues advancing with new therapeutic options
❌ Dangerous Assumption
“My cholesterol is genetic, so there’s nothing I can do about it.”
✅ Empowering Truth
Even genetic high cholesterol responds dramatically to proper treatment. Familial hypercholesterolemia patients can achieve 50-70% LDL reductions with appropriate therapy, preventing most cardiovascular events.
The future of cholesterol management promises even more personalized approaches, incorporating genetic insights, advanced biomarkers, and novel therapeutic targets. However, the fundamental principles remain clear: early identification, comprehensive risk assessment, lifestyle optimization, and appropriate medical therapy when indicated can dramatically reduce the burden of cardiovascular disease and improve lives.
“The goal isn’t to eliminate cholesterol entirely—it’s to optimize the balance between beneficial and harmful lipoproteins to support long-term cardiovascular health while maintaining quality of life.”
Whether you’re a patient newly diagnosed with high cholesterol, a healthcare provider seeking current best practices, or someone interested in preventive health strategies, the evidence is clear—optimal cholesterol management is one of the most powerful tools available for protecting cardiovascular health and ensuring a longer, healthier life.
References
- Feingold KR. Guidelines for the Management of High Blood Cholesterol. Endotext. Updated March 27, 2025.
- 2025 Consensus on the Clinical Pathway of Blood Cholesterol Management in Taiwan. PMC. February 15, 2025.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019.
- Mayo Clinic. High cholesterol – Symptoms and causes. March 7, 2025.
- Cleveland Clinic. Hyperlipidemia (High Cholesterol): Levels, Causes, Symptoms & Diagnosis. June 2, 2025.
- Goldberg IJ, Sharma G, Fisher EA. The Role of Lipids and Lipoproteins in Atherosclerosis. Endotext. January 3, 2019.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering. JACC. 2022.
- Lee Y, Siddiqui WJ. Cholesterol Levels. StatPearls. Updated July 24, 2023.
- Patel SB, Wyne KL, Afreen S, et al. 2025 AACE Clinical Practice Guideline for the Pharmacologic Management of Adults with Dyslipidemia. Endocrine Practice. February 2025.
- Harvard Health Publishing. Can you reduce your cholesterol without taking a drug? August 1, 2024.
- American Heart Association. What is Atherosclerosis? November 16, 2017.
- Gidding SS, Allen NB. Cholesterol and Atherosclerotic Cardiovascular Disease: A Lifelong Problem. Journal of the American Heart Association. 2019.
Related Articles
- Natural Remedies vs Prescription Drugs: What’s the Difference?
- Can Oatmeal Lower Cholesterol? The Science Behind Oats vs. Statins
- Lower Cholesterol Supplements that actually work
- High Blood Pressure: Effective Management Strategies
- Which is Better for Diabetes, Berberine or Metformin?
- Lowering Cholesterol Diet Tips and Tricks
Disclaimer: This article is for educational purposes only and should not replace professional medical advice. Always consult with a qualified healthcare provider before making changes to your cholesterol management plan or starting new treatments. Individual responses to interventions may vary, and what works for one person may not be appropriate for another.
Image Credits:
Hero — “Artery with Atherosclerosis Progression” via Wikimedia Commons (CC BY-SA 4.0).
“LDL Receptor Pathway Endocytosis” by Mikael Häggström via Wikimedia Commons (CC BY-SA 3.0).
“LDL Particle with ApoB-100” illustration by David S. Goodsell, RCSB PDB-101 (CC BY 4.0 / educational use).
“Rosuvastatin (Crestor) Tablets” via Wikimedia Commons (CC BY 4.0).
“HMG-CoA to Mevalonate Pathway” diagram via Wikimedia Commons (CC BY-SA 4.0).
“Mediterranean Diet Pyramid” via Wikimedia Commons (CC BY-SA 3.0).
All other images are in the public domain or used under open-license educational terms.